Study Definition
Notification Service
Drug Supply Management
Online randomisation
Data Management
ALEA Clinical is an internet service provided by ALEA Clinical Services that supports online patient registration, randomisation, (predictive) drug supply management and eCRF.
Used by Pharmaceuticals & Academics
Software to optimize your clinical trial

ALEA eCRF service
ALEA eCRF is an electronic Case Report Forms service for data collection in clinical trials. It is a comprehensive, user friendly forms service which can be used with a standard browser running on any computer connected to the internet. The current version is suitable for virtually any trial in health care. Within ALEA eCRF, the components share a database for storing and retrieving information about the trial, and a separate database for storing and retrieving patient data.
Improve Patient Randomisation
The randomisation service includes flexible form definition, unlimited stratification factors, unlimited number of treatments, blocked randomisation, minimisation, unequal allocation schemes, multi step randomisation, a simulation service which produces a comprehensive validation report and more. It allows for re-use of components at the level of questions, forms and complete studies.
Create notifications
The Notification Service Module is responsible for the distribution of notifications after randomisation. These may be sent by email, fax, or printed directly on a printer attached to the randomisation server network. Most notifications are triggered by the randomisation of a patient into the trial and such notifications may be sent to, for example, the responsible clinician, a study monitor at the sponsoring organisation (a pharmaceutical company), or a pharmacist who will prepare the study medication. As well as notifications are send after the randomisation, the Notification Service Module can distribute periodic status reports on the trial or generate reports after specific events within the trial.
Manage Forms Anywhere
ALEA eCRF is an electronic Case Report Forms service for the data collection in clinical trials. It provides a comprehensive, user friendly forms service which can be used in a standard browser running on any computer connected to the internet. ALEA has been built as a series of industry grade components from Microsoft, which have been customized for the specific purpose of clinical trials data management. ALEA is suitable for virtually any trial and is used in over 700 trials and 7.000 sites worldwide.
Software That Researchers Will Embrace
ALEA has been built as a series of components, in order to support interoperability and customized extensions. The components make use of a common standard respresentation of data and metadata: the Operational Data Model of CDISC.
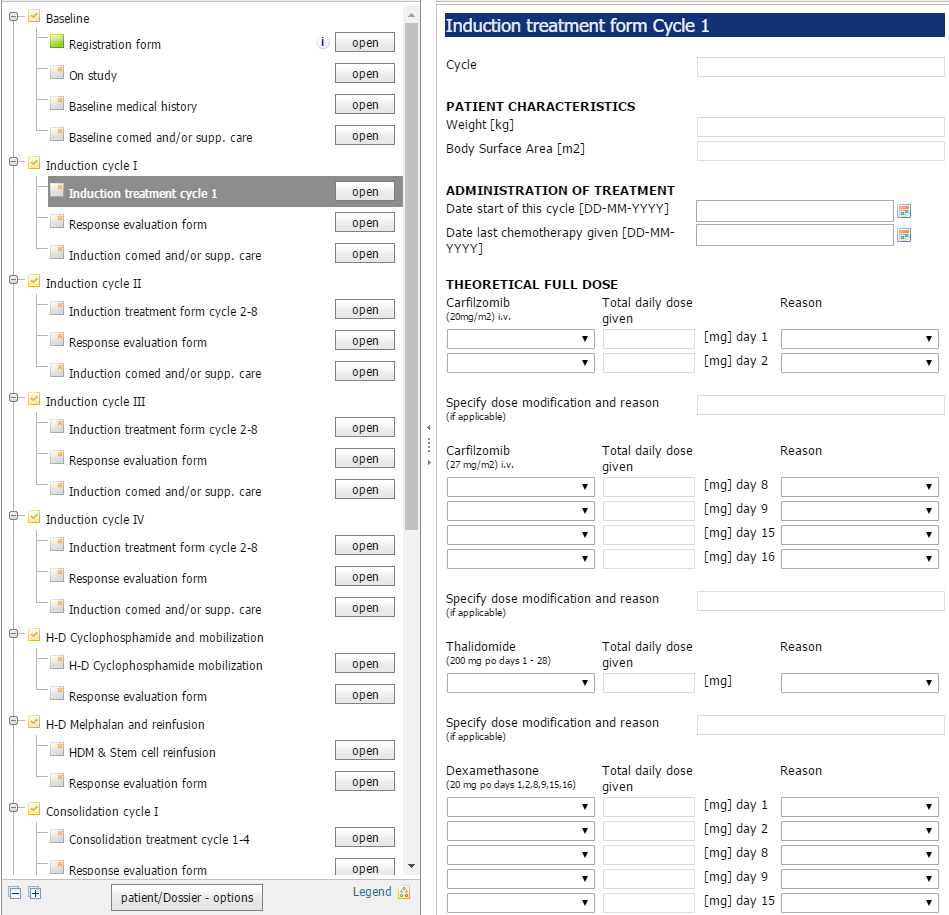
Software That Researchers Will Embrace
ALEA has been built as a series of components, in order to support interoperability and customized extensions. The components make use of a common standard respresentation of data and metadata: the Operational Data Model of CDISC.


Full Service
“The ALEA services not only benefit from rigorous academic input during their development but also reflect an understanding of the needs of academic research. Their licensing has allowed Institut Bergonie to pool its TENALEA instance with collaborating institutes without additional charges.''
- Pr Simone Mathoulin-Pelissie - Institut Bergonie, Bordeaux